Describe How Physical And Chemical Changes Are Different. How Are They Similar?
Chemical Alter vs. Concrete Change
- Page ID
- 346
The difference between a physical reaction and a chemical reaction is composition. In a chemical reaction, there is a change in the composition of the substances in question; in a physical change there is a deviation in the appearance, smell, or elementary display of a sample of matter without a modify in composition. Although nosotros call them physical "reactions," no reaction is actually occurring. In order for a reaction to take place, there must be a alter in the elemental composition of the substance in question. Thus, nosotros shall only refer to physical "reactions" equally physical changes from at present on.
Introduction
Physical changes are limited to changes that upshot in a departure in brandish without changing the composition. Some common changes (but not express to) are:
- Texture
- Color
- Temperature
- Shape
- Change of Land (Boiling Indicate and Melting Point are significant factors in determining this alter.)
Physical properties include many other aspects of a substance. The post-obit are (but not express to) physical properties.
- Luster
- Malleability
- Ability to be fatigued into a thin wire
- Density
- Viscosity
- Solubility
- Mass
- Volume
Any change in these physical properties is referred to equally a physical change. For further information, please refer to Backdrop of Affair.
Chemical changes, on the other hand, are quite different. A chemic change occurs when the substance'southward limerick is changed. When bonds are broken and new ones are formed a chemic alter occurs. The post-obit are indicators of chemic changes:
- Change in Temperature
- Change in Color
- Noticeable Aroma (after reaction has begun)
- Germination of a Precipitate
- Formation of Bubbles
Note: When two or more reactants are mixed and a alter in temperature, color, etc. is noticed, a chemical reaction is probably occurring. These are non definite indicators; a chemical reaction may non be occurring. A change in colour is non always a chemical change. If 1 were to change the colour of a substance in a not-chemic reaction scenario, such as painting a car, the change is physical and non chemical. This is considering the composition of the car has not changed. Continue with caution.
Common Physical Changes
Texture
The texture of a substance can differ with a concrete change. For instance, if a piece of wood was sanded, waxed, and polished, information technology would have a very unlike texture than it initially had every bit a crude piece of forest.

(left) Rough plank boardwalk, Quebec City, Canada (correct) Finished mountain ash flooring. (CC BY-SA 4.0; WikiPedant and CC BY-SA 2.5; MarkAnthonyBoyle, respectively).
As y'all can see, the texture of the finished wood is much smoother than the initial grainy wood.
Color
The irresolute of colour of a substance is non necessarily an indicator of a chemical change. For example, changing the color of a metallic does not change its physical properties. Still, in a chemic reaction, a color alter is commonly an indicator that a reaction is occurring. Painting the metal car does not changing the composition of the metal substance.
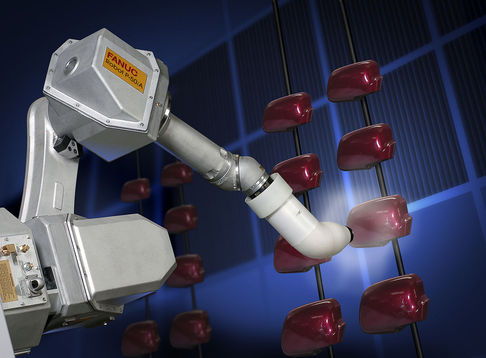
Robotic arm applying pigment on motorcar parts. Image use with permission (CC BY-SA 4.0l RoboGuru).
Temperature
Although we cannot see temperature alter, unless if a change of state is occurring, it is a physical change.

Hot metalwork. (CC BY-SA-NC 2.0; flagstaffotos.com.au)
One cannot meet the pan physically changing shape, color, texture, or any of the other concrete backdrop. However, if one were to touch the pan, information technology would be incredibly hot and could cause a burn. Sitting idle in a closet, this pan would be cold. One cannot appraise this change only through visual exposure; the use of a thermometer or other instrument is necessary.
Shape
The shape of an object tin can be inverse and the object will still remain true to its chemical composition. For example, if one were to fold money, equally shown by the figure below, the money is yet chemically the same.
Origami Coin

Alter of State
The change of state is likewise a physical change. In this scenario, one tin can observe a number of physical properties irresolute, such as viscosity and shape. Every bit ice turns into water, it does not retain a solid shape and now becomes a sticky fluid. The physical "reaction" for the change of ice into liquid water is:
\[H_2O_{(s)} \rightarrow H_2O_{(fifty)}\]

The following are the changes of state:
| Solid → Liquid | Melting |
| Liquid → Gas | Vaporization |
| Liquid → Solid | Freezing |
| Gas → Liquid | Condensation |
| Solid → Gas | Sublimation |
- If oestrus is added to a substance, such as in melting, vaporization, and sublimation, the process is endothermic. In this instance, rut is increasing the speed of the molecules causing them move faster.
- If heat is removed from a substance, such every bit in freezing and condensation, then process is exothermic. In this case, rut is decreasing the speed of the molecules causing them move slower.
Physical Properties
Luster
The luster of an element is divers as the style it reacts to calorie-free. Luster is a quality of a metallic. Near all of the metals, transition metals, and metalloids are lustrous. The not-metals and gases are not lustrous. For case, oxygen and bromine are not lustrous. Shown below is are lustrous paper clips.
Lustrous Paperclips

Malleability
Malleability is also a quality of metals. Metals are said to exist malleable. This ways that the metals can deform under an amount of stress. For case, if you lot tin can hitting a metal with a mallet and it deforms, information technology is malleable. Also, a paperclip can be shaped with bare hands.
Bent Paperclip

The image shows the malleability of a sure metal as stress is applied to it.
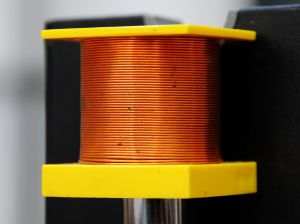
Power to be drawn into a thin wire
In materials science, this property is called ductility. For case, raw copper can be obtained and it can be purified and wrapped into a cord. Once again, this belongings is feature of mainly metals, nonmetals exercise not possess this quality.
Copper Wire
Density
The density of an object is its mass divided by its volume (d=chiliad/v). A substance will have a higher density if it has more mass in a fixed amount of volume. For example, accept a brawl of metallic, roughly the size of a baseball, compressed from raw metallic. Compare this to a baseball game made of paper. The baseball made of metal has a much greater weight to information technology in the same amount of volume. Therefore the baseball fabricated out of metal has a much higher density. The density of an object volition also determine whether it volition sink or float in a detail chemical. Water for example has a density of 1g/cmiii. Any substance with a density lower than that will bladder, while any substance with a density above that will sink.
Oil Sinking in a Glass of Water

Viscosity
Viscosity is defined to be the resistance to deformation of a item chemical substance when a forcefulness is applied to it. In the example beneath, 1 tin see ii cubes falling into 2 different test tubes. The upper substance shows a violent reaction to the dropping of the cube. The lower substance only engulfs it slowly without much reaction. The upper substance has a lower viscosity relative to the lower substance, which has very high viscosity. One may even retrieve of viscosity in terms of thickness. The substance with more thickness has higher viscosity than a substance that is deemed "thin." Water has a lower viscosity than beloved or magma, which have relatively loftier viscosities.
Viscosity of Fluids

Common Chemical Changes
The follow are all indicators of chemical reactions. For further data on chemical reactions, please refer to Chemical Reactions.
Modify in Temperature
A change in temperature is characteristic of a chemical alter. During an experiment, ane could dip a thermometer into a beaker or Erlenmeyer Flask to verify a temperature change. If temperature increases, as it does in most reactions, a chemical change is probable to be occurring. This is different from the concrete temperature alter. During a physical temperature change, one substance, such as h2o is being heated. However, in this case, i chemical compound is mixed in with another, and these reactants produce a product. When the reactants are mixed, the temperature modify caused by the reaction is an indicator of a chemical change.

As an example of a exothermic reaction, if \(Fe_2O_3\) is mixed with Al and ignighted (often with burning Mg), and then the thermite reaciton is initiated
\[Fe_2O_3 + 2Al \rightarrow 2Fe + Al_2O_3 + \text{Heat}\]
This reaction generates heat as a production and is (very) exothermic.
However, physical changes can be exothermic or endothermic. The melting of an ice cube, which is endothermic, is a change in a physical holding and not composition. Thus, information technology is a physical change.
Change in Colour
A change in color is too another characteristic of a chemical reaction taking place. For example, if one were to notice the rusting of metal over time, one would realized that the metal has changed color and turned orange. This modify in color is evidence of a chemical reaction. Even so, one must be conscientious; sometimes a change in color is only the mixing of two colors, but no real change in the composition of the substances in question.
Metallic Rusting

The reaction above is that of the rusting of atomic number 26.
\[4Fe + 3O_2 + 6H_2O \rightarrow 4Fe(OH)_3\]
Noticeable Odor
When ii or more compounds or elements are mixed and a scent or aroma is present, a chemic reaction has taken identify. For case, when an egg begins to aroma, (a rotten egg) a chemical reaction has taken place. This is the result of a chemical decomposition.
Spoiled Egg

Formation of a Precipitate
The formation of a precipitate may be i of the most common signs of a chemic reaction taking place. A precipitate is defined to be a solid that forms within of a solution or some other solid. Precipitates should not be confused with suspensions, which are solutions that are homogeneous fluids with particles floating almost in them. For instance, when a soluble carbonate reacts with Barium, a Barium Carbonate precipitate tin exist observed.
Examination Tube

Reaction:
\[Ba^{2+}_{(aq)} + CO^{ii-}_{3\;(aq)} \rightarrow BaCO){3\;(southward)}\]
For further data, please refer to Nomenclature of Thing.
Formation of Bubbles
The germination of bubbling, or rather a gas, is another indicator of a chemic reaction taking place. When bubbles form, a temperature modify could also be taking identify. Temperature change and formation of bubbles frequently occur together. For example, in the following paradigm, ane can see a gas spewing. This is the formation of a gas.
Gas Germination

However, virtually reactions are much more than subtle. For instance, if the following reaction occurs, i may find Carbon Dioxide bubbling forming. If there is enough Hydrochloric acid, bubbles are visible. If there isn't, one can't readily notice the modify:
\[Na_2CO_3 + 2HCl \rightarrow 2NaCl + H_2O + CO_2\]
References
- Chang, Raymond. General Chemistry: the Essential Concepts. Boston, MA: McGraw-Colina Higher Pedagogy, 2006. Print.
- Chemistry for Dummies. For Dummies, 2008. Print.
- Petrucci, Ralph H. Full general Chemistry Principles and Modern Applications. Upper Saddle River, NJ: Pearson/Prentice Hall, 2007. Print.
Exterior Links
All images are courtesy of http://www.sxc.hu, which provides royalty free images that are free to be copied without restrictions. The viscosity image is as well free to be duplicated as per permission of author on Wikipedia.com.
Issues
1. Which of the following is a chemical reaction?
- Freezing liquid Mercury
- Adding xanthous to blue to brand green
- Cutting a slice of newspaper into two pieces
- Dropping a sliced orange into a vat of Sodium Hydroxide
- Filling a balloon with natural air
ii. Which of the following is a physical reaction?
- Shattering Glass with a baseball
- Corroding Metal
- Fireworks Exploding
- Lighting a match
- Baking a block
3. Which of the following is a chemical reaction?
- Painting a wall blue
- A bicycle rusting
- Ice cream melting
- Scratching a primal beyond a desk-bound
- Making a sand castle
4. Which of the post-obit is a concrete reaction?
- Frying an egg
- Digesting carrots
- A Macbook falling out of a window
- Creating ATP in the human body
- Dropping a fizzy tablet into a drinking glass of water
5. Write C for Chemical Reaction or P for Physical Reaction.
- Burning Leaves
- Cutting Diamonds
- Crushing a pencil
- The salivary amylase enzyme that breaks downward nutrient in the rima oris
- Table salt mixing in with water
Answers
1. D
ii. A
3. B
4. C
5. a) C
b) P
c) P
d) C
east) Neither. This is i of the gray areas of chemical modify and physical change. Although the common salt has dissociated into Sodium and Chloride ions, it is still table salt in water. Salt, initially is actually but a conglomerate of sodium and chloride ions and by dissociating them, merely the arrangement of the ions has changed. Delight click hither for more information.
Describe How Physical And Chemical Changes Are Different. How Are They Similar?,
Source: https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_%28Physical_and_Theoretical_Chemistry%29/Fundamentals/Chemical_Change_vs._Physical_Change
Posted by: russbrisiong.blogspot.com


0 Response to "Describe How Physical And Chemical Changes Are Different. How Are They Similar?"
Post a Comment